Covalent Bonds Diagram
Asimo-tech: covalent Covalent bond: definition, types, and examples Polar covalent bonds
polarity | Definition & Examples | Britannica
Dot and cross diagrams for simple covalent molecules (i) Covalent bonds Functions and structures of dna and nucleotide
Covalent bonding molecule asimo tech methane concepts arose kind early
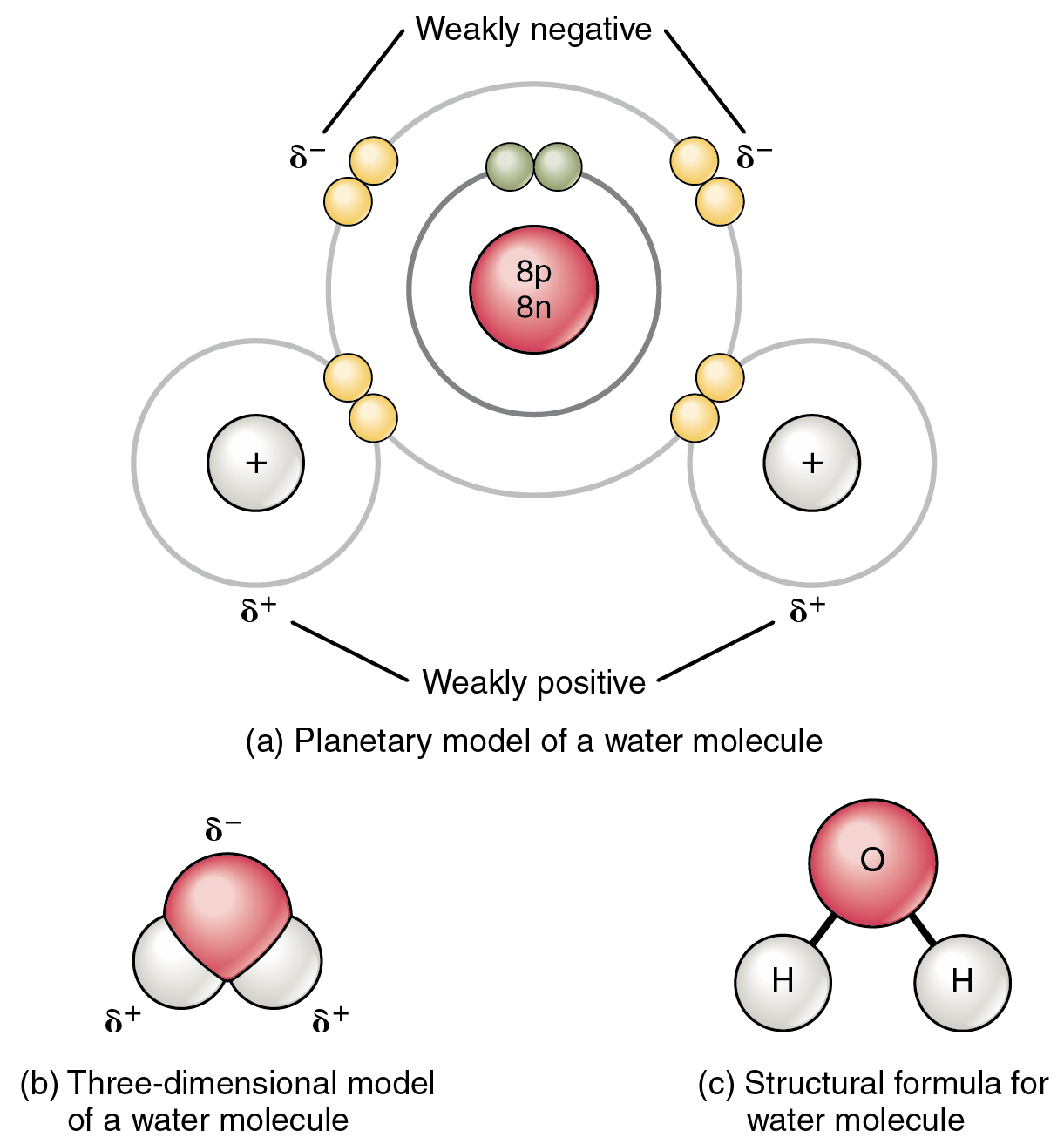
Polar covalent bonds polarity ikatan kovalen nonpolar materikimia molecule atoms hydrogen oxygen molecules chemical electrons h2o britannicaCovalent bond ionic vs bonds chemistry structure chemical elements ck12 nc license cc ck saved Covalent bondBonds covalent forces attractive intramolecular atoms electrons.
What are the types of covalent bonds?Bonding chemical types crystal bonds bond molecular chemistry ionic covalent metallic britannica crystals theory different compounds intermolecular forces nodal techniques Covalent bond: definition, types, and examplesChemical bonding.

Covalent bonds bond formed electrons between atoms two valence nonmetals chemical which ppt pair powerpoint presentation always
Ammonia covalent nh3 chemical bonding struktur molekul nitrogen ch4 ammonium bentuk nitrate nitrite hydrogen molecule ionic elektron electron refrigeration valencaCovalent bonds bond chemical types hydrogen four ionic atoms between molecule two online fs Bonds structure phosphodiester nucleotide backbone nucleic molecule acids ester nucleotides phosphate covalent together form nitrogenous bases bonding hydroxyl carbons microbiologyCovalent molecules compounds chemistry molecular bonds elements figure part.
In the dna backbone, which bonds exactly are considered ester bondsBonding and structure Covalent dna bonds nucleotide onlineCovalent bond polar bonds.

Ch150: chapter 4 – covalent bonds and molecular compounds – chemistry
License : cc by-nc 3.0Bonding covalent bond dot cross structure molecular bonds simple compounds gcse double triple atom each there electrons Covalent bonds biology molecules atomsCovalent polar bonds bond ionic nonpolar chemistry lewis electron bonding electrons structures using.
Attractive forces and bondsCovalent molecules hydrogen oxygen chlorine Lewis structure ammonia covalent bond lone pair chemical bond, png.